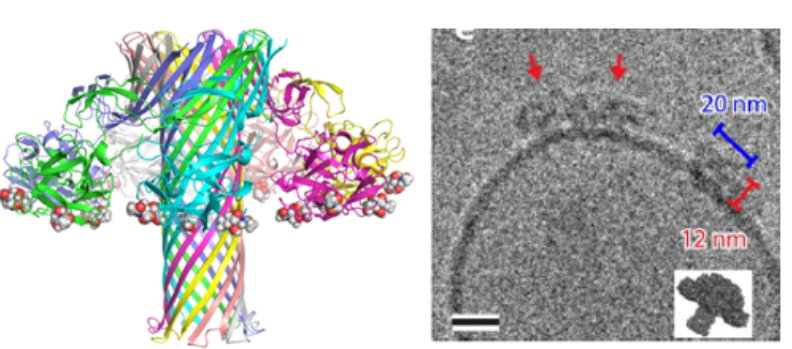
As part of the Unilectin web service, the TrefLec database contain β-trefoil candidate lectins from more than 4000 species. Looking for combination of lectin and toxin domains resulted in the identification of SaroL-1 protein sequence, from primitive colony forming eukaryote Salpingoeca rosetta. Sarol-1 has a lectin domain with affinity for cancer-related α-galactosylated epitopes such as the glycosphingolipid Gb3 and an aerolysin domain able to oligomerize while forming pore in membranes. Combination of x-ray crystallography and cryo-electron microscopy allowed for elucidating the carbohydrate-dependent pore-forming function of SaroL-1
The article is available (open source) over here.