Abstract:
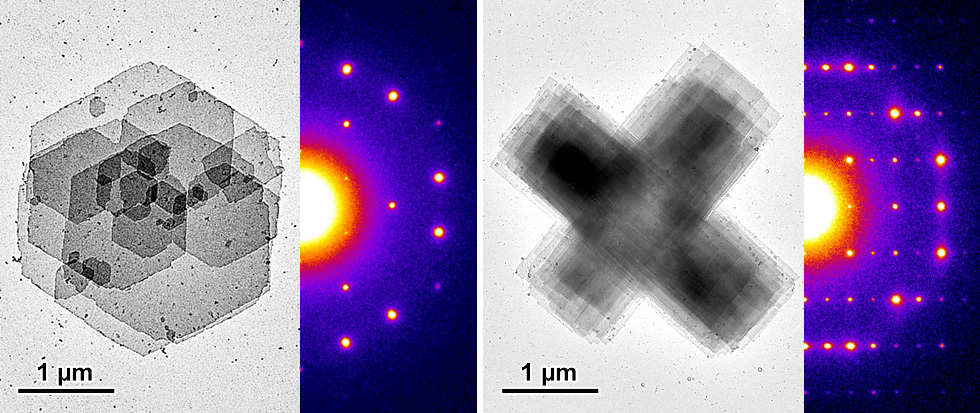
Chain-folded lamellar crystalline complexes of amylose with aliphatic diols were prepared and characterized by transmission electron microscopy as well as electron and X-ray diffraction. Four allomorphs were identified, depending on the complexing diol and crystallization conditions, that consisted of orthorhombic lattices of antiparallel 6- or 7-fold amylose single helices. Straight-chain n-diols (ethane-1,2-diol, butane-1,4-diol, hexane-1,6-diol) yielded 6-fold helical complexes regardless the length of the linear chain, whereas a bulkier branched diol (2 methylpentane-2,4-diol) induced the formation of 7-fold helices. Butane-1,3-diol, that contains a straight carbon chain with one end and one side hydroxyl group, induced both 6- and 7-fold helical conformations depending on the crystallization conditions. When a diol induced more than one allomorph, an adequate control of its concentration and crystallization temperature allowed targeting a specific crystalline form. Upon drying, all allomorphs were converted into more compact pseudo-hexagonal structures. The dried form of a given complex generally retained the same helical conformation as the hydrated one.
The article is availaible over here.