Abstract:
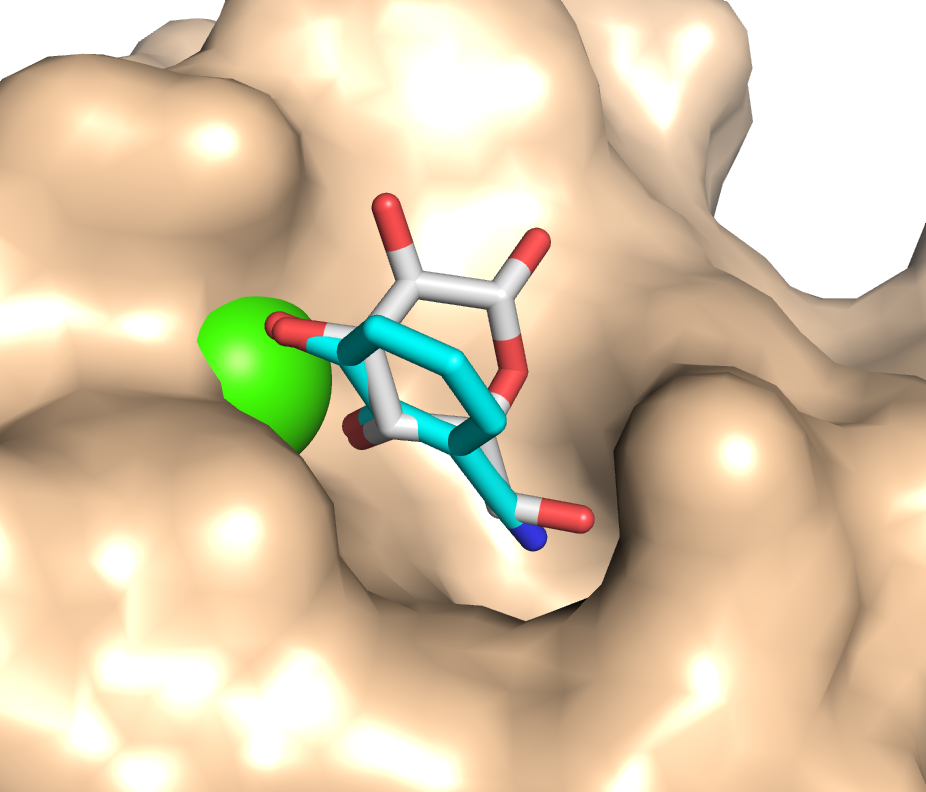
« Because of the antimicrobial resistance crisis, lectins are considered novel drug targets. Pseudomonas aeruginosa utilizes LecA and LecB in the infection process. Inhibition of both lectins with carbohydrate‐derived molecules can reduce biofilm formation to restore antimicrobial susceptibility. Here, we focused on non‐carbohydrate inhibitors for LecA to explore new avenues for lectin inhibition. From a screening cascade we obtained one experimentally confirmed hit, a catechol, belonging to the well‐known PAINS compounds. Rigorous analyses validated electron‐deficient catechols as millimolar LecA inhibitors. The first co‐crystal structure of a non‐carbohydrate inhibitor in complex with a bacterial lectin clearly demonstrates the catechol mimicking the binding of natural glycosides with LecA. Importantly, catechol 3 is the first non‐carbohydrate lectin ligand that binds bacterial and mammalian calcium(II)‐binding lectins, giving rise to this fundamentally new class of glycomimetics. »
The article is availaible over here.