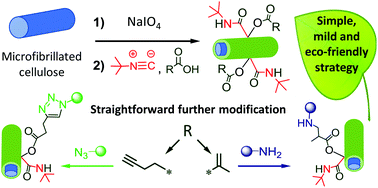
Abstract:
“Through a cascade of chemical derivatizations involving the green Passerini three-component reaction (P-3CR), we describe herein the multifunctionnalization of cellulose microfibrils (MFC) previously subjected to a periodate oxidation step. Not only do MFC constitute a remarkably attractive cellulose substrate from industrial and fundamental standpoints, but their periodate oxidation increases their reactivity while keeping their unique attributes. The Passerini reaction allowed for the successful grafting of two judiciously chosen chemical precursors in aqueous one-pot heterogeneous conditions, thus leading to the generation of dually modified (both functional and reactive) MFCs. Namely, as a proof of concept of this strategy, a tert-butyl isocyanide and a carboxylic acid, the latter bearing either an alkyne or a methacrylate function, were reacted with the aldehyde moieties present at the surface of periodate oxidized cellulose (POC). A thorough characterization evidenced the success of the double functionnalization, provided the degrees of substitution (DS) and allowed for elucidating the impact of structural (i.e. degree of oxidation of POC, nature of the acid partner) and experimental (i.e. amount of reagent) parameters on the P-3CR yield. Finally, the reactive Passerini-modified MFCs were conveniently post-derivatized by appealing aqueous coupling ligations. Specifically, MFC decorated with methacrylate functions were reacted with an amine through the aza-Michael reaction, while the propensity of alkyne-functionalized MFC to react through copper catalyzed Huisgen cycloaddition was demonstrated by grafting two different azides. This P-3CR chemical strategy exhibiting good atom economy appears as a mild, versatile, and eco-friendly synthetic approach for the dual surface modification of MFCs. Furthermore, the facility to post-modify such reactive Passerini-modified MFC objects through various popular ligation reactions paves the way to the straightforward generation of a large panel of tailor-made MFC objects in an aqueous process, whose surface properties can be suitably adjusted to the targeted application by the right choice of the two precursors engaged in P-3CRs.”
The article is available over here: https://pubs.rsc.org/en/content/articlelanding/2020/gc/d0gc02532a#!divAbstract