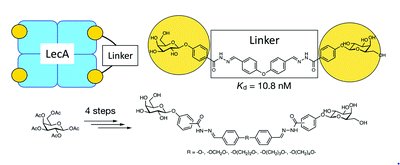
Chronic infections with Pseudomonas aeruginosa are associated with the formation of bacterial biofilms. The tetrameric P. aeruginosa lectin LecA is a virulence factor and an anti-biofilm drug target. Increasing the overall binding affinity by multivalent presentation of binding epitopes can enhance the weak carbohydrate–ligand interactions. Low-nanomolar divalent LecA ligands/inhibitors with up to 260-fold valency-normalized potency boost and excellent selectivity over human galectin-1 were synthesized from D-galactose pentaacetate and benzaldehyde-based linkers in four linear steps.
Our publication is availaible here:
https://pubs.rsc.org/en/content/articlehtml/2020/cc/d0cc03490h