Abstract:
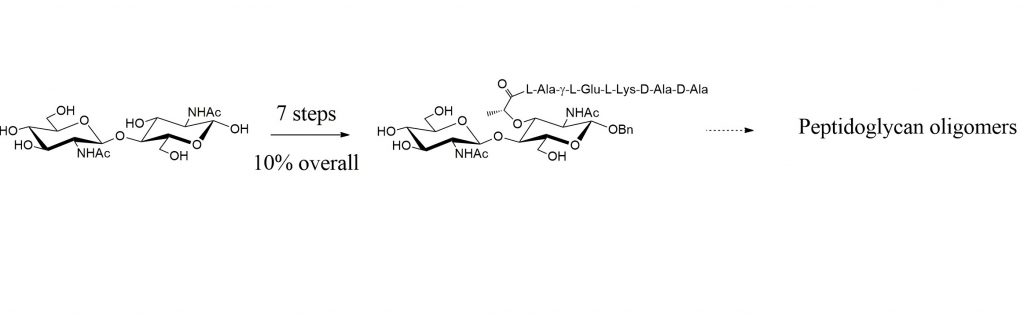
« Peptidoglycan oligomers constitute precious tools for the biochemical and structural studies of enzymes involved in the bacterial cell wall metabolism. In this study we show that an unprecedented selective Zemplén de-O-acetylation of benzyl chitinbioside peracetate leads to a fast and efficient route to N-acetylmuramyl β(1→4) N-acetylglucosaminide disaccharide, a central building block for the synthesis of peptidoglycan oligomers. Starting from known benzyl chitinbioside, NAG-NAM disaccharide pentapeptide is prepared in seven steps with an overall yield of 12%. »
The paper is available (open access) over here.