Abstract:
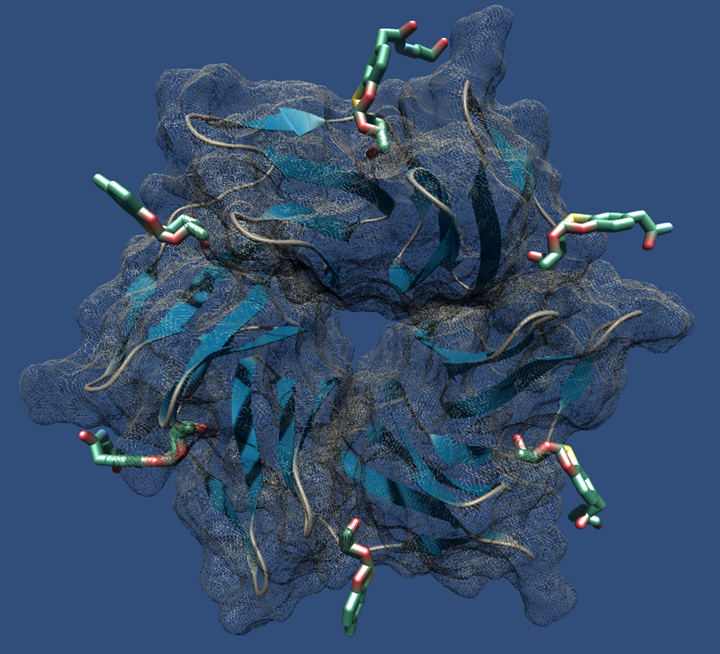
“Two orthogonal, metal free click reactions, enabled to glycosylate ubiquitin and its mutant A28C forming two protein scaffolds with high affinity for BambL, a lectin from the human pathogen Burkholderia ambifaria. A new fucoside analogue, with high affinity with BambL, firstly synthetized and co-crystallized with the protein target, provided the insights for sugar determinants grafting onto ubiquitin. Three ubiquitin-based glycosides were thus assembled. Fuc-Ub, presented several copies of the fucoside analogue, with proper geometry for multivalent effect; Rha-A28C, displayed one thio-rhamnose, known for its ability to tuning the immunological response; finally, Fuc-Rha-A28C, included both multiple fucoside analogs and the rhamnose residue. Fuc-Ub and Fuc-Rha-A28C ligands proved high affinity for BambL and unprecedented immune modulatory properties towards macrophages activation.”
The article is availaible over here: https://pubs.rsc.org/en/content/articlelanding/2020/sc/d0sc03741a#!divAbstract