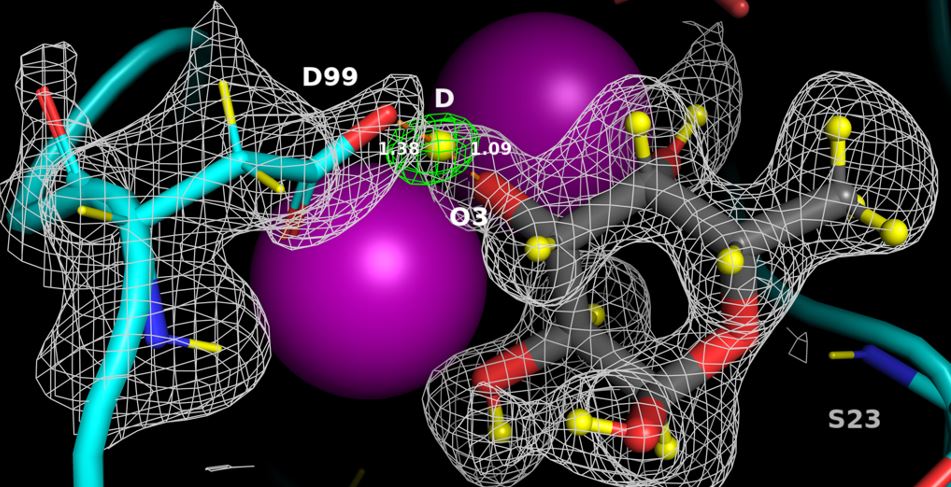
Pseudomonas aeruginosa , bacterium involved in hospital-acquired infections, has been identified by the World Health Organization as a critical priority for new antibiotics. Lukas Gajdos, a PhD student co-supervised by CERMAV and ILL teams, deciphered the mechanisms involved in the binding of this pathogen to host cells thanks to neutron crystallography. The perdeuteration of the LecB lectin and fucose ligand, and the neutron structure of the complex allowed for identifiying the positions of all the hydrogen atoms. The strong binding affinity is related to the occurrence of a low-barrier hydrogen bond induced by the proximity of the two calcium ions, the presence of coordination rings between the sugar, calcium and LecB, and the dynamic behaviour of bridging water molecules at room temperature. These key structural details may assist in the design of anti-adhesive compounds to combat multi-resistance bacterial infections.
The article is available over here.