The Structural and Molecular GlycoBiology (GBMS) team seeks to dissect, at the atomic scale, the interactions of carbohydrates with the proteins involved in their synthesis, degradation or recognition. Its research is at the Chemistry-Biology interface with multidisciplinary expertise in bioinformatics, molecular biology, biochemistry and enzymology, X-ray protein crystallography, analysis of protein-carbohydrate interactions and molecular modelling.
The main research axes of the team are:
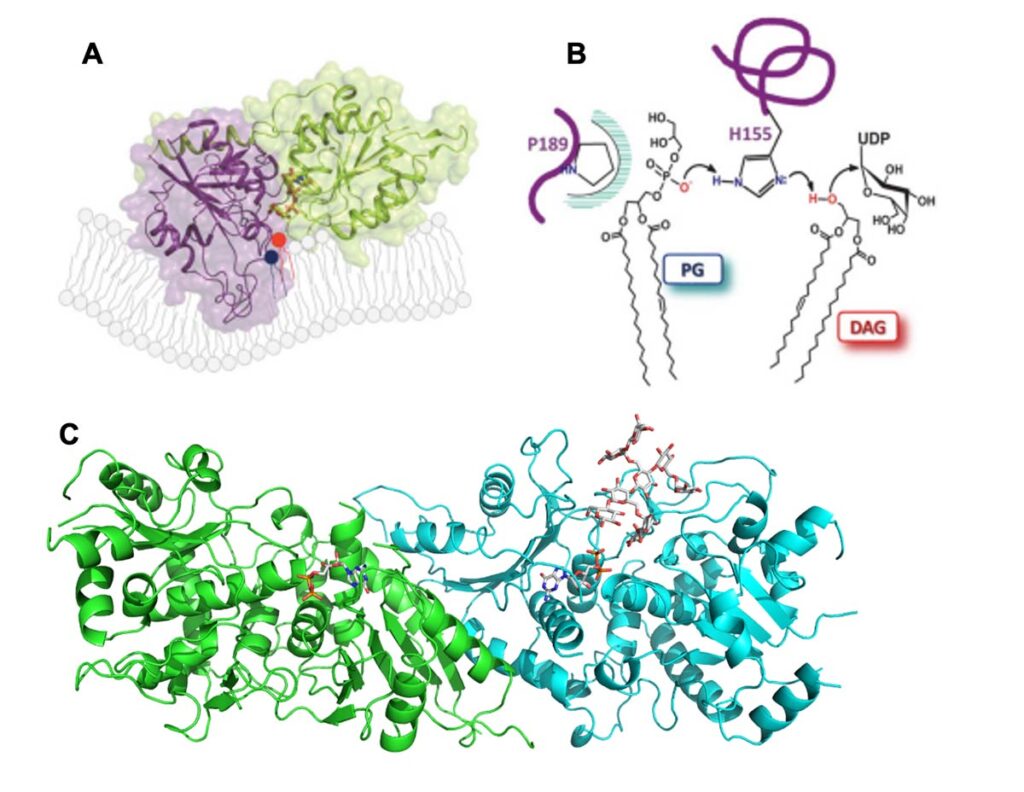
The biosynthesis of complex carbohydrates by glycosyltransferases (GTs): C. Breton
Glycosylation is the most important biochemical reaction on Earth and GTs are the key enzymes in this process. It is therefore important to progress in the knowledge of their mechanism of action and their regulation. We focus our efforts on the study of GTs involved in the biogenesis of galactolipids, essential compounds of photosynthetic membranes and of the plant cell wall with the synthesis of xyloglucan as a study model.
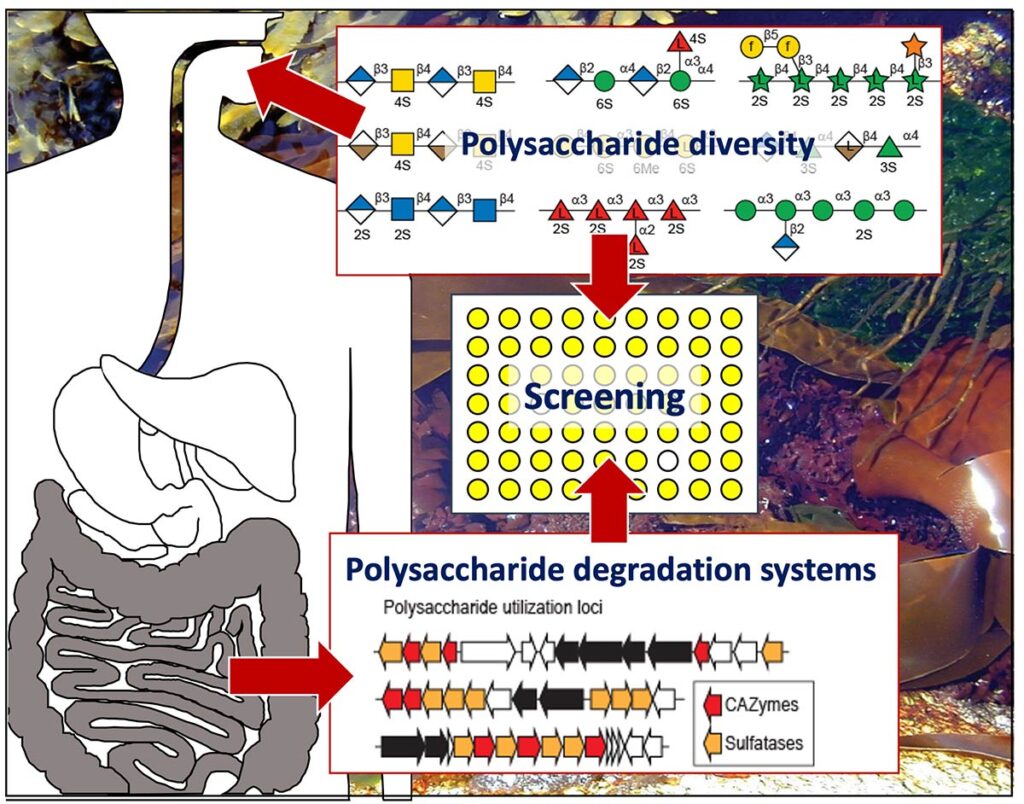
Degradation of polysaccharides by PULs: W. Helbert, S. Drouillard & M. Couturier
Through the study of Polysaccharide utilization loci (PULs), we contribute to the global effort to assign the function of genes coding for putative enzymes for the degradation of complex polysaccharides and to understand their degradation. We focus on PULs identified in bacterial and fungal genomes of human gut microbiota potentially involved in the degradation of plant polysaccharides and sulfated polysaccharides of marine origin. The newly discovered enzymes can be new tools for the structural characterization of polysaccharides or for the production of oligosaccharides as potential bioactive compounds. The studies of the mode of action and specificity of the most original enzymes are carried out using modern enzymological approaches (chromatography, NMR and mass spectrometry). Thus, we maintain and enrich a unique collection of approximately 200 oligo- and polysaccharide substrates of which the structure of the most originals is analyzed in detail using the PCAN service (CERMAV) and the NMR platform (ICMG).
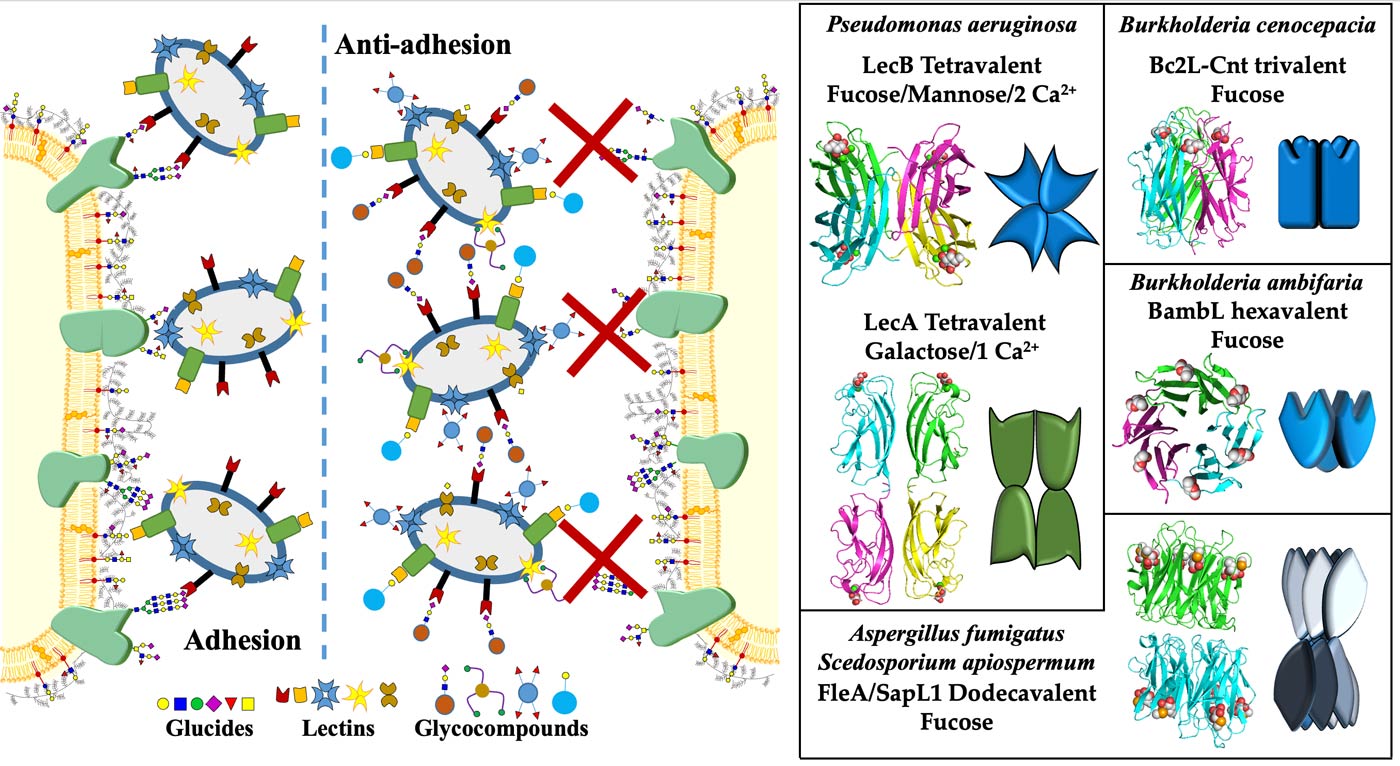
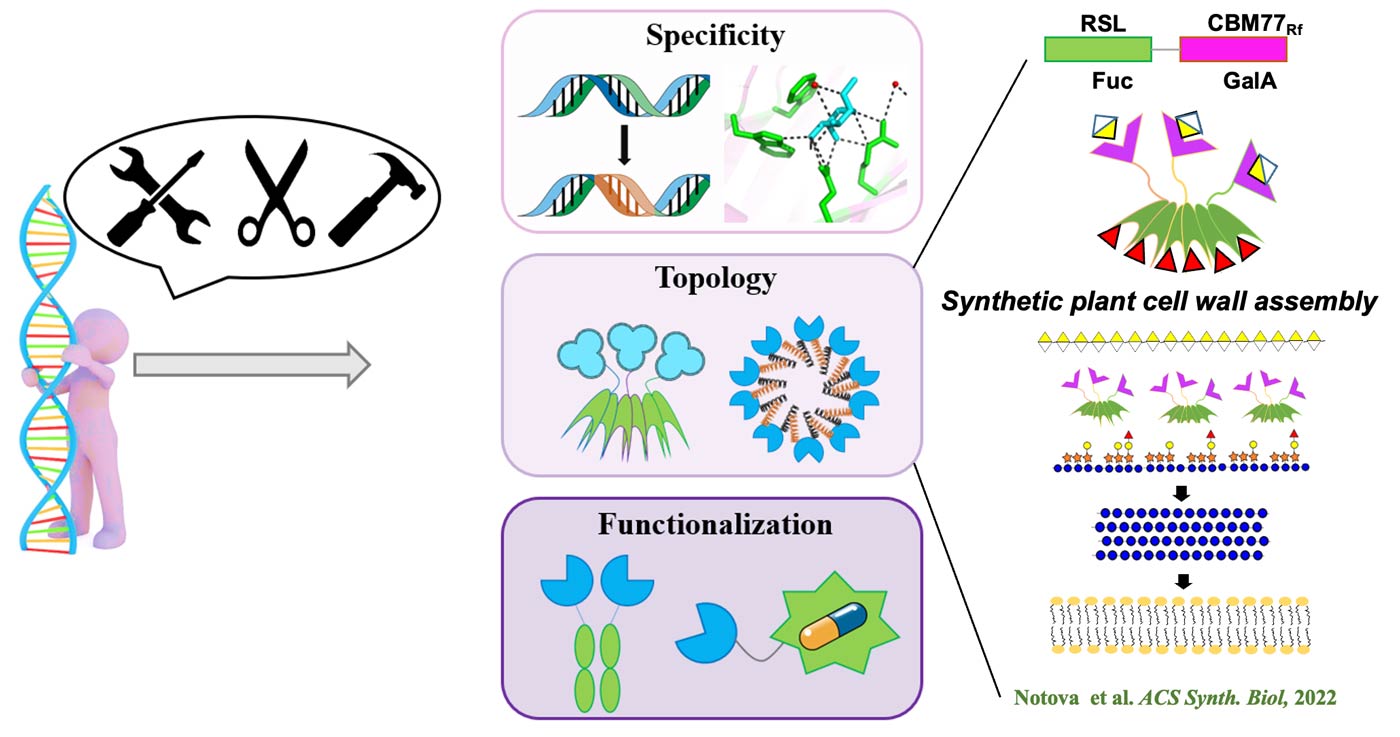
Recognition of glycoconjugates by lectins: A. Imberty & A. Varrot
Lectins are protein receptors capable of deciphering the glycocode. They play an essential role in host-pathogen interactions, either as a first-line defense molecule against foreign organisms, or by promoting microbial adhesion, a crucial step in the initiation of infections. Lectins are now targets for the development of antiadhesive glycocompounds that could be used as anti-infectives. Through their recognition of specific carbohydrate motifs, they are also sought-after molecular tools to aid in the diagnosis and prognosis of cancers often associated with glycosylation aberrations and for controlling the glycosylation of biotherapeutic products. We are also interested in the identification of new lectins both in terms of their folding or their specificity, in obtaining the atomic details governing carbohydrate-protein interactions and in the engineering of lectins by synthetic biology to modify their specificity or multivalence and create new tools. We have a catalog of about sixty lectins or lectin domains of various origins characterized and available in recombinant form and 110 structures deposited in the Protein Data Bank.
The GBMS team is involved in making glycosciences better known. It develops tools and databases: Glyco3D and Unilectin and participates in the dissemination of knowledge in Glycopedia . It develops new graphic approaches to visualize (SweetUnityMol), study but also teach glycosciences.
The GBMS team is a partner of the CDP Glyco@Alps2 within the framework of the IDEX Université Grenoble Alpes (UGA), the Labex Arcane and the ITN “GlycoNoVi” funded by the HORIZON-MSCA-DN-2021 action. It participates in several Cost actions: CM1102, CA18132 and CA1803. It is currently supported by the National Agency for Research through the projects: LectArray, SweetDisplay, S-Plore, CAZyMYC and WITT, by the association Vaincre la Mucoviscidose, UGA and CNRS.
The GBMS team (january 2025)
Permanent staff
- Christelle Breton, Prof. – UGA
- Valérie Chazalet, Assistant Engineer – CNRS
- Marie Couturier, Researcher – CNRS
- Sophie Drouillard, Assitant Professor – UGA
- Emilie Gillon, Assistant Engineer – CNRS
- William Helbert, Director of Research – CNRS
- Anne Imberty, Director of Research – CNRS
- Serge Pérez, Director of Research Emeritus – CNRS
- Mélanie Touvrey-Loiodice, CNRS
- Annabelle Varrot, Director of Research – CNRS (team leader)
Non permanent staff
- Théodore Arnaud, PhD Student
- Flavien Dedieu, Research engineer
- Palig Karaminassian, PhD Student
- Batoul Moubarak, PhD Student
- Manon Quillerrou Moreau, PhD Student
- Luca Pisapla, PhD Student